Human Auditory Nerve
Secret hearing misfortune (HHL) is an as of late depicted hear-able nephropathy accepted to add to discourse separation and clarity deficiencies in individuals with ordinary audiological tests. Creatures and people with HHL have typical hear-able edges yet blemished cochlear neurotransmission, that is, decreased suprathreshold adequacy of the sound-evoked hear-able nerve compound activity potential. Presently, the lone cell system known for HHL is the loss of internal hair cell neurotransmitters (synaptopathy). Here we report that transient loss of cochlear Schwann cells brings about perpetual hear-able deficiencies normal for HHL. This hear-able neuropathy isn't related to synaptic misfortune, yet rather with interruption of the first heminodes at the hear-able nerve fringe terminal. Hence, this examination distinguishes another component for HHL, features the long haul outcomes of transient Schwann cell misfortune on hearing, and may give bits of knowledge into the reasons for the hear-able deficiencies announced in patients that recuperate from intense demyelinating infections like Guillain–Barré condition.
Analysis Of The Human Auditory Nerve
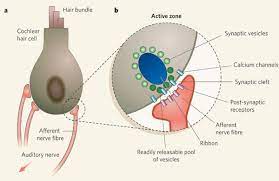
In the mammalian cochlea, acoustic upgrades are distinguished by internal hair cells (IHCs), which transduce and change them into synaptic signs to the essential hear-able tangible neurons, the twisting ganglion neurons (SGNs). These neurons then transfer the acoustic data to the focal hear-able circuits. Along these lines, the legitimate capacity of both tactile cells and neurons is fundamental for ordinary hearing and hear-able correspondence, and degeneration of either part causes sensorineural hearing misfortune. This kind of hearing misfortune, which influences 320 million individuals worldwide 1, is portrayed by the lasting rise of hear-able limits, that is, the negligible sound pressing factor levels (SPLs) that summon a hear-able or neural reaction at explicit sound frequencies. As of not long ago, it was accepted that hear-able handling is typical in subjects with ordinary limits, even after recuperation from a transitory edge shift (TTS) because of commotion openness, and that hearing shortfalls in those cases were because of focal problems2. Notwithstanding, late creature and human examinations demonstrate that moderate clamor openings or maturing can bring about another type of fringe hearing misfortune called 'covered up hearing misfortune (HHL)'3,4,5,6. HHL can be recognized physiologically and is portrayed by ordinary hear-able edges however diminished suprathreshold abundancy of the sound-evoked SGN compound activity potential (AP) (the main pinnacle of the hear-able brainstem reaction (ABR) waveform)3,5,6,7,8 and by the change in the proportion between the pinnacle of the ABR waveform created by hair cells (summating potential (SP)) and the pinnacle I of the ABR waveform (AP)9. The decreased neuronal initiation seen in HHL has been proposed to bring about the debasement of the transient coding of suprathreshold sounds and deficiencies in discourse segregation and comprehensibility, especially in a loud environment10,11; in any case, the last is not symptomatic of HHL, as they could be likewise brought about by focal preparing shortfalls. HHL has been proposed to prompt hyperactivation of focal hear-able neural connections, subsequently conceivably adding to tinnitus3,12. As of recently, misfortune or brokenness of IHC neural connections has been the solitary proposed system of HHL13. Here we report an extra cell component for HHL
ear canal structure.
Hearing System
In the cochlea, tangible hair cells and neurons are in close relationship with a few sorts of glial and glia-like cells14. Hair cells are encircled by supporting cells, SGN axons are myelinated by Schwann cells, and twisting ganglion cell bodies are wrapped by satellite cells (Fig. 1a). In earlier examinations, we investigated the jobs of supporting cells in IHC neurotransmitter development and regeneration15 and the conservation of hair cells16. As a feature of our progressing endeavors to characterize the jobs the various populaces of cochlear glia play in hearing and deafness15,16,17, we examined the outcomes of Schwann cell removal in the developing cochlea. We tracked down that intense Schwann cell misfortune causes fast hear-able nerve demyelination, which is trailed by strong Schwann cell recovery and axonal remyelination. Shockingly, this transient Schwann cell misfortune brings about a lasting hear-able weakness normal for HHL. Nonetheless, this HHL contrasts from that seen after clamor openness or maturing in that it happens without adjustments in synaptic thickness but instead connects with particular and lasting interruptions of the first heminodes at the hear-able nerve axon near the IHCs. Besides, the two kinds of HHL happen autonomously (clamor openings that cause HHL don't disturb heminodes) and they are added substances. Together, these outcomes uncover another component for the pathogenesis of HHL and another result of myelin absconds on the typical capacity of the sensory system.
Vestibular system
As expected from earlier investigations of mice with DTA-incited removal of Plp1-communicating cells19, tamoxifen intraperitoneal (i.p.) infusion to mice conveying Plp1/CreERT transgene and Rosa26DTA alleles [DTA(+/−): Plp1/Cre(+/−)] from P21 to 23 brought about awkward step and rear appendage loss of motion somewhere in the range of 1 and fourteen days post-DTA articulation followed by complete recuperation of ordinary stride by 3 a month post-DTA articulation. This is predictable with the vigorous regenerative limit of Plp1-communicating glia cells19. Curiously, although DTA acceptance at P35 brings about critical mortality 3 weeks after the fact (ref. 19 and our unpublished information), we found that removal of Plp1-communicating cells at P21 doesn't bring about lethality. These perceptions recommend that the wellbeing impacts of deficiency of Plp1+ cells increment with age.
Comments
Post a Comment